Diagnosis of an active CMV infection
Complete kit for the rapid diagnosis of an active CMV infection in 4 hours
Features
- Detection of CMV pp65 positive PMNs by indirect immunofluorescence gives high sensitivity and easy reading of the results
- A cocktail of antibodies specific for pp65
- A complete kit containing all the reagents needed from cell preparation to immunofluorescence staining
- The number of CMV pp65 positive cells are counted per duplicate stain
- No evidence of cross reactivity between other viruses and CMV using the CMV antigenemia assay with the C10/C11 monoclonal antibody cocktail
- Control slides are included
- Completed within 5 hours of sample collection
- The kit is available in a 100 tests version
- Registered as Medical Device for In Vitro Diagnostic Use (IVD/CE0344)
- FDA cleared (USA) 510(k) #951550
Applications
- Bone marrow transplant recipients
- Solid organ transplant patients
- HIV infected persons
- AIDS patients
For in vitro diagnostics use only. Not for use in humans.
Order now!
Order your products via our order form!
Product code: VIR-CMV 100
(Please note that this product will be discontinued as of 1-1-2022. The alternative product is the CMV Brite Turbo kit.)
Introduction
CMV infection in a healthy human is usually subclinical. However, in the immuno-compromised host and a developing foetus it may result in either a localized or disseminated disease. Clinical manifestations of CMV disease include pneumonia, retinitis, hepatitis, enteritis and neurological disease. Despite improved treatment modalities, CMV infection may result in significant morbidity and mortality. Patients are at risk from both primary CMV infection and reactivation of latent infection.
The CMV pp65 antigenemia assay is a valuable tool in the diagnosis and monitoring of active CMV infection in solid organ and bone marrow transplant patients as well as in the diagnosis and monitoring of CMV disease in AIDS patients. Early and rapid diagnosis of active CMV infection is of great importance in avoiding over-treatment with immunosuppressive drugs and in guiding antiviral therapy.
The standard in CMV testing, antigenemia, is a non-culture technique that detects an active infection with blood sample analysis and is optimized for use in the CMV Brite™ Kit and CMV Brite™ Turbo Kit.
The CMV Brite™ antigenemia kit uses the well defined C10/C11 antibody cocktail to detect the CMV lower matrix phosphoprotein (pp65), an early antigen in virus replication, which is abundantly present in antigen-positive polymorphonuclear cells.
Principle of the CMV Brite™ Kit
The CMV antigenemia assay has been developed using a cocktail of two monoclonal antibodies (C10/C11) directed against CMV lower matrix protein pp65. The assay uses the C10/C11 cocktail in an indirect immunofluorescence staining of cytospin preparations of peripheral blood leukocytes.
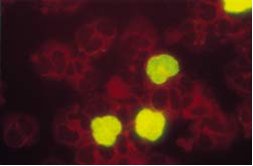
Figure: Human peripheral blood leukocytes from a patient with an active CMV infection, stained with the CMV Brite antigenemia kit. Immunofluorescence staining of CMV pp65 antigen positive polymorph nuclear cells. Negative cells are counterstained (red) with Evans Blue.
- Landry,M.L. et al. Evaluation of CMV Brite Kit for detection of Cytomegalovirus pp65 antigenemia in peripheral blood leucocytes by immunofluroescence. J.Clin.Microbiol. 34. 1337-1339
- Van Son, W.J., The,T.H., (1989) Cytomegaovirus infection after organ transplantation: an update with special emphasis on renal transplantation. Transpl. Int. 1989 Oct; 2(3), 147-164
- The, T.H., et al, Cytomegalovirus antigenemia. Rev Infect Dis. 1990 Sep-Oct;12 Suppl 7:S734-44.
- Van der Bij et al., (1988) Comparison between viremia and antigenemia for detection of cytomegalovirus in blood. J. Clin. Microbiology 26. 2531-2535
- Grefte, J.M.M et al (1992) The lower matrix protein pp65 is the principal viral antigen present in peripheral blood leukocytes during an active cytomegalovirus infection. J.Gen. Virol. 73. 2923-2932
Package Insert
English/German/French/Spanish/Italian: